Abstract
Introduction: Intermediate-risk acute myeloid leukemia (IR-AML) is a clinically heterogeneous disease, for which optimal post-remission therapy has been debated. Several studies using next-generation sequencing have identified important prognostic information and led to modifications in risk stratification. However, the utility of this genetic information in decision making for IR-AML has yet to be elucidated. We investigated the clinical relevance of recurrent driver gene mutations in a well-characterized cohort of IR-AML patients in first complete remission (CR1) after induction chemotherapy.
Methods: A total of 1,589 AML patients treated at the Cleveland Clinic (CC) between 2002 and 2016 were screened for eligibility, of whom 825 were in European Leukemia Net (ELN) IR category. Of them, 100 IR-AML patients, who had mutational information at diagnosis and achieved CR after ≥ 1 round of intensive induction were included. CR was defined as <5% bone marrow blasts, absolute neutrophil counts ≥ 1x109/L, and platelet counts ≥ 100x109/L. CR with inadequate count recovery (CRi) was same as CR, except for residual neutropenia or thrombocytopenia. The induction regimen was 7+3, and patients with persistent disease at day 14 marrow were re-induced with 7+3 or 5+2. Patients who did not receive intensive therapy or achieve CR/CRi were excluded. A TruSeq Custom Amplicon panel targeting exons of 62 genes was used for deep sequencing at diagnosis. We used The Cancer Genome Atlas (TCGA) data for validation of our findings.
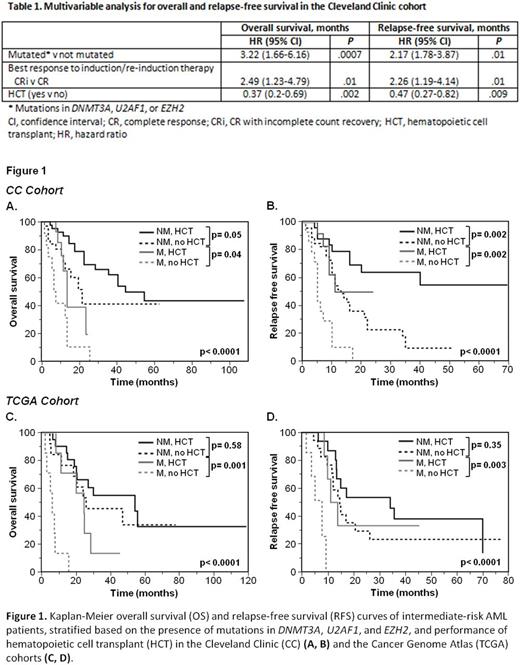
Results: In the CC cohort, median age was 58.5 (24-75) years, 64% had normal cytogenetics, and 31% required >1 induction cycles to achieve CR1. DNMT3A and FLT3-ITD mutations were the most common (19%), followed by RUNX1 (15%), TET2 (12%), IDH2 (12%), NPM1 (12%), ASXL1 (11%), and U2AF1 (11%). The frequency of persistent disease after initial induction was higher in patients with mutations in DNMT3A (47% vs 27%, p= .03) and U2AF1 (55% vs 28%, p= .01), while CRi was more common in patients with BCOR mutation (50% vs 8.5%, p= .02). In univariable analysis, patients with mutations in DNMT3A, U2AF1, and EZH2, and patients who achieved CRi after induction had worse overall (OS) and relapse-free survival (RFS). Patients who had hematopoietic cell transplant (HCT) in CR1 achieved better OS (HR, 0.4; 95% CI, 0.2-0.7; p=0.003) and RFS (HR, 0.3; 95% CI, 0.1-0.6; p< 0.001) than those who did not. Other clinical factors analyzed, such as age, sex, cytogenetics, and the number of induction courses to achieve CR1 were not significant predictors for survival after CR1. To evaluate the impact of these three mutations on risk stratification, we compared patients whose AML harbored any of the mutations with those who did not. The median OS and RFS for patients with mutations were 13 and 9 months, respectively, compared to 41 and 16 months in patients without (p< 0.0001 and p= .003). Similarly, TCGA patients with mutations had significantly poorer OS (median, 9.8 vs 46.8 months, p= .0002) and RFS (median, 9.3 vs 17 months, p= .02). In multivariable analysis, mutations maintained an independent effect on survival (Table 1).
Since mutations and HCT were the major predictors of outcome after CR1, we analyzed the outcomes of patients harboring mutations in relation to HCT (Figure 1). In both cohorts, patients who did not have the mutations and underwent HCT had the best outcomes. In the CC cohort, median OS and RFS for patients who had mutations and HCT were 14 and 11 months, respectively, compared to 7.5 and 5 months in patients who did not undergo HCT (p= .04 for OS and p= .002 for RFS). In the TCGA cohort, patients who harbored mutations and did not undergo HCT had significantly worse OS (median, 6.3 vs 24.6 months, p= .001) and RFS (median, 7.8 vs 12.5 months, p= .003). In view of the recently proposed ELN 2017 criteria that place IR patients with RUNX1 or ASXL1 mutations into the adverse risk, we explored outcomes of these patients at the time of CR1. Presence of these mutations did not predict OS and RFS in our cohort, and performance of HCT did not confer a significant survival benefit in patients with these mutations.
Conclusions: In two independent cohorts, DNMT3A, U2AF1, and EZH2 mutations were predictors of relapse and OS in IR-AML at CR1, and performance of HCT translated into survival benefit. Our results provide evidence of clinical utility in considering mutation screening to stratify IR-AML patients to guide therapeutic decisions.
Sekeres: Celgene: Membership on an entity's Board of Directors or advisory committees. Gerds: CTI BioPharma: Consultancy; Incyte: Consultancy. Maciejewski: Alexion Pharmaceuticals, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fees; Ra Pharma: Consultancy; Apellis Pharmaceuticals: Consultancy. Advani: Pfizer: Consultancy; Takeda/ Millenium: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.